
Providing $8.3 Billion for Coronavirus Response, Preparedness, & Vaccine Development (H.R. 6074)
Do you support or oppose this bill?
What is H.R. 6074?
(Updated January 18, 2022)
This bill would provide a total of $8.3 billion in funding to respond to the coronavirus, including vaccine development, financial support for state and local governments, and assistance for affected small businesses. A breakdown of its various provisions can be found below.
Centers for Disease Control & Prevention (CDC): The CDC would receive a total of $2.2 billion to support federal, state, and local public health agencies to prevent, prepare for, and respond to the coronavirus, including:
$950 million, of which $475 million would have to be allocated within 30 days, to support states, localities, territories, and tribes in coronavirus surveillance; laboratory testing to detect positive cases; contact tracing to identify additional positive cases; infection control at the local level to prevent additional cases; migration in areas with person-to-person transmission to prevent additional cases; and other preparedness & response activities.
$300 million to replenish the Infectious Diseases Rapid Response Reserve Fund, which supports immediate response activities during outbreaks.
At least $300 million for global disease detection and emergency response.
This bill would also reimburse state & local costs incurred for coronavirus preparedness and response activities between January 20 and this bill’s date of enactment. It would also allow funds to be used for the construction or renovation of facilities to improve preparedness and response capabilities at the state and local level.
Vaccines, Therapeutics, & Diagnostics: This section would provide over $3 billion for the research & development (R&D) of vaccines, therapeutics, and diagnostics to prevent or treat the effects of coronavirus, including:
Over $2 billion for the Biomedical Advanced Research and Development Authority (BARDA) to support advanced R&D of vaccines, therapeutics, and diagnostics, prioritizing platform-based technologies with U.S.-based manufacturing capabilities.
$826 million for the National Institutes of Health (NIH) to support basic R&D of vaccines, therapeutics, and diagnostics.
$300 million in contingency funding for procurement of vaccines, therapeutics, and diagnostics.
Additionally, this section would require that vaccines, therapeutics, and diagnostics developed using taxpayer funds be available for purchase by the federal government at a fair and reasonable price. It would also allow the Secretary of Health & Human Services (HHS) to ensure that vaccines, therapeutics, and diagnostics developed using taxpayer funds be affordable in the commercial market.
Healthcare Preparedness, Pharmaceuticals & Medical Supplies: This section would provide $1 billion for procurement of pharmaceuticals and medical supplies to support healthcare preparedness and Community Health Centers, and to improve medical surge capacity, including:
About $500 million for the procurement of pharmaceuticals, masks, personal protective equipment, and other medical supplies which can be distributed to state & local health agencies in areas with a shortage of supplies.
$100 million for health services through Community Health Centers, which will support smaller health clinics across the country in under-served urban & rural areas.
A continuation of healthcare preparedness support, including the National Ebola & Special Pathogens Training & Education Center (NETEC), regional, state & local special pathogens treatment centers, and hospital preparedness cooperative agreements.
Funding for medical surge capacity, which will increase the supply of biocontainment beds at additional health facilities.
A requirement to reimburse $136 million to HHS programs that had funding temporarily transferred to support emergency preparedness & response activities at the CDC and the Assistant Secretary for Preparedness and Response.
$10 million for worker-based training to prevent & reduce exposure of hospital employees, emergency first responders, and other workers who are at risk of exposure to coronavirus through their work duties.
$2 million for HHS to conduct oversight of activities related to coronavirus preparedness & response.
Authority for HHS to hire public health experts as expeditiously as necessary to perform critical coronavirus-related work.
This bill would allow HHS to waive certain Medicare telehealth restrictions during the coronavirus public health emergency. These waivers would allow Medicare providers to provide telehealth services to beneficiaries regardless of whether the beneficiary is in a rural community, and would also allow beneficiaries to receive care from physicians & other practitioners in their homes. This provision has an estimated cost of $500 million.
Food & Drug Administration (FDA): This section would provide the FDA with $61 million to facilitate the development and review, both pre- & post-market, of medical countermeasures, devices, therapies, and vaccines to combat the coronavirus. It would help to maintain the national drug & device product inventory through extensive outreach to manufacturers to identify potential supply chain interruptions; assist enforcement work against counterfeit & misbranded products and its review of emergency use authorizations for medical products, such as diagnostics.
Additionally, this section will enable the FDA to build on its efforts to strengthen U.S. medical product manufacturing by supporting efforts to foster more investment and innovation in advanced manufacturing methods for drugs, devices, vaccines, and other therapies.
Small Business Administration (SBA): This section would allow the SBA to provide up to $1 billion in loan subsidies to help small businesses, small agricultural cooperatives, small aquaculture producers, and non-profit organizations which have been impacted by financial losses as a result of the coronavirus outbreak. This funding could enable the SBA to provide an estimated $7 billion in loans to these entities. Additionally, $20 million would be provided to administer these loans.
Miscellaneous
The State Dept. would receive $264 million for consular operations, emergency evacuations of staff & dependents, and other emergency preparedness needs at embassies around the world. It would also receive $435 million to support health systems overseas; $300 million to respond to humanitarian needs arising in countries with a coronavirus outbreak; $250 million to protect against the effects of an outbreak including economic, security, and stabilization requirements.
The president would be restricted from using funds appropriated in this bill for any other purpose, except for repayment of transfers within HHS. The Government Accountability Office (GAO) would be required to provide enhanced oversight of funds appropriated in this bill.
Argument in favor
This bipartisan funding package will provide much-need resources to respond to the coronavirus outbreak and develop therapeutics for treatment & vaccines for prevention in the future.
Argument opposed
Congress has taken far too long to develop this funding package to respond to the coronavirus outbreak because of their desire to score political points.
Impact
People affected by the coronavirus outbreak; healthcare providers; federal, state, and local public health agencies; pharmaceutical companies.
Cost of H.R. 6074
The CBO estimates that enacting this bill would cost $7.570 billion over the 2020-2030 period.
Additional Info
In-Depth: House Appropriations Committee Chairwoman Nita Lowey (D-NY) offered the following statement on the introduction of this package to address the coronavirus:
“The American people are counting on our government for a full-funded, coordinated, and comprehensive government-wide response to the coronavirus. House Democrats’ emergency supplemental consists of robust, entirely new funding and strong transparency and accountability measures to fully address the virus and keep Americans safe from this growing public health emergency. We must quickly enact this legislation -- lives are at stake.”
House Minority Leader Kevin McCarthy (R-CA) tweeted a criticism of the process employed by House Democrats in reaching the agreement:
“Congress should’ve already passed a funding bill to combat coronavirus. Period. But Democrats are hellbent on sneaking in elements of their liberal agenda — which would slow down vaccine development and availability. We need CLEAN funding. No gimmicks. No new strings attached.”
Senate Appropriations Committee Chairman Richard Shelby (R-AL) delivered remarks on the Senate floor following the compromise that led to this package’s introduction:
“We listened carefully to the agencies and experts on the front lines in crafting this package. Vice President Pence has been very helpful in this effort, and I appreciate President Trump’s eagerness to sign this legislation. I also want to thank Leaders McConnell and Schumer, Vice Chairman Leahy, Chairwoman Lowey, and Ranking Member Granger for coming together to the right thing for the American people. We face this crisis together; we are fighting it together. Ultimately, I believe we will prevail together. But now is the time for action.”
President Donald Trump initially requested $2.5 billion to address the coronavirus outbreak but after Democrats said they felt a figure closer to $8 billion was needed, the president signaled his willingness to sign whatever funding package Congress sends to his desk:
“With respect to the money that’s being negotiated, they can do whatever they want. We’re requesting $2.5 [billion]. Some Republicans would like us to get $4 [billion], and some Democrats would like us to get $8.5 [billion], and we’ll be satisfied whatever it is.”
Media:
Roll Call (Context)
Summary by Eric Revell
(Photo Credit: iStock.com / gevende)
The Latest
-
 The Long Arc: Taking Action in Times of Change“Change does not roll in on the wheels of inevitability, but comes through continuous struggle.” Martin Luther King Jr. Today in read more... Advocacy
The Long Arc: Taking Action in Times of Change“Change does not roll in on the wheels of inevitability, but comes through continuous struggle.” Martin Luther King Jr. Today in read more... Advocacy -
 Thousands Displaced as Climate Change Fuels Wildfire Catastrophe in Los AngelesIt's been a week of unprecedented destruction in Los Angeles. So far the Palisades, Eaton and other fires have burned 35,000 read more... Environment
Thousands Displaced as Climate Change Fuels Wildfire Catastrophe in Los AngelesIt's been a week of unprecedented destruction in Los Angeles. So far the Palisades, Eaton and other fires have burned 35,000 read more... Environment -
 Puberty, Privacy, and PolicyOn December 11, the Montana Supreme Court temporarily blocked SB99 , a law that sought to ban gender-affirming care for read more... Families
Puberty, Privacy, and PolicyOn December 11, the Montana Supreme Court temporarily blocked SB99 , a law that sought to ban gender-affirming care for read more... Families -
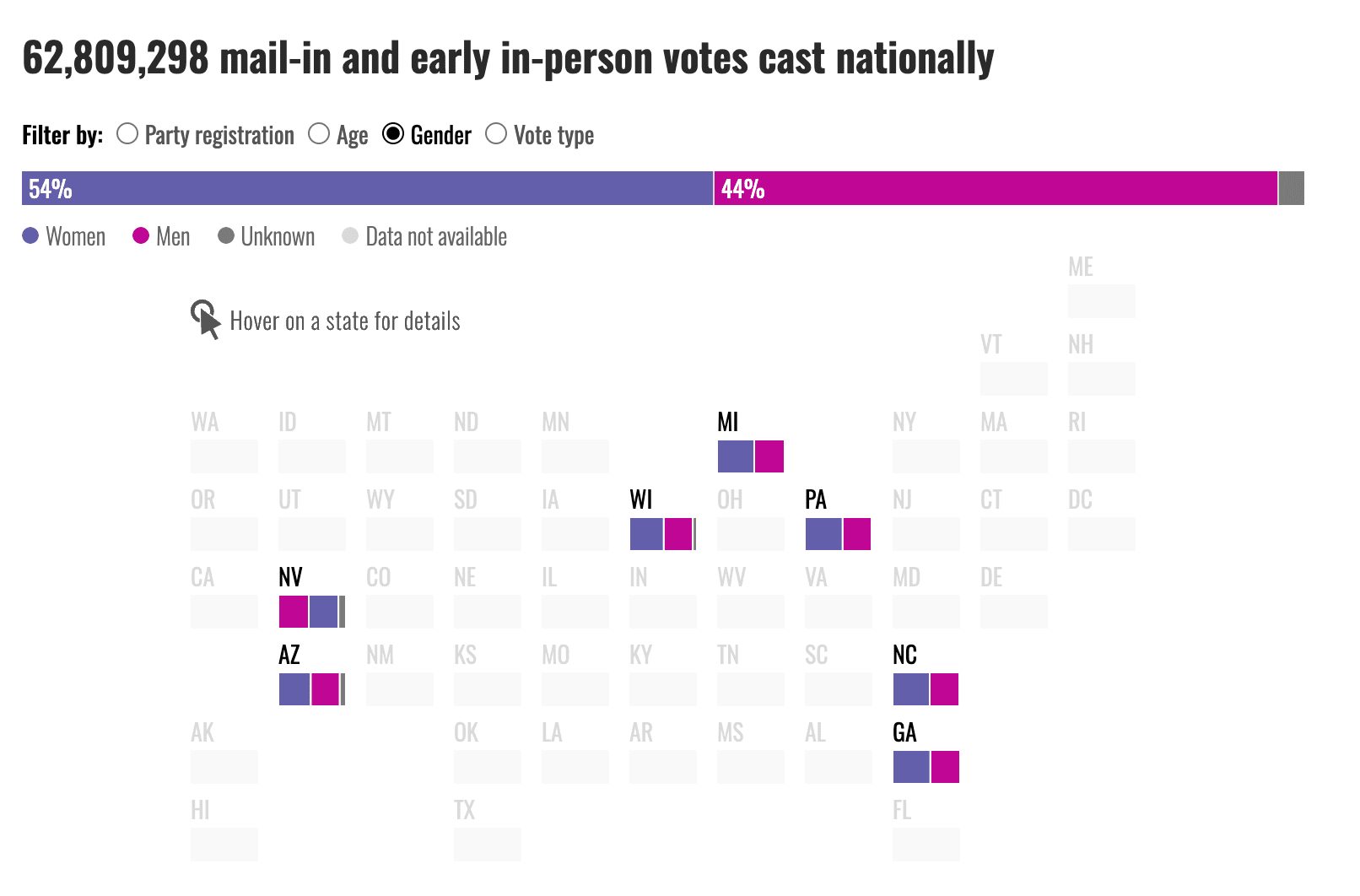 Women Are Shaping This Election — Why Is the Media Missing It?As we reflect on the media coverage of this election season, it’s clear that mainstream outlets have zeroed in on the usual read more... Elections
Women Are Shaping This Election — Why Is the Media Missing It?As we reflect on the media coverage of this election season, it’s clear that mainstream outlets have zeroed in on the usual read more... Elections
 Climate & Consumption
Climate & Consumption
 Health & Hunger
Health & Hunger
 Politics & Policy
Politics & Policy
 Safety & Security
Safety & Security
